 |
 |
|
|
| Real-time monitoring of cellular signaling pathways: Development of fluorescent biosensors for cellular second messengers. |
 |
 |
| Real time observation of messenger molecules in individual intact cells
is essential for physiological studies of signaling mechanisms. The signaling
cascades to link extracellular messengers to intracellular Ca2+ mobilization
are regulated by the second messenger D-myo-inositol- 1,4,5-trisphosphate
(IP3). A direct metabolite of IP3, D-myo-inositol-1,3,4,5-tetrakisphosphate
(IP4), is also believed to be a pivotal second messenger in cellular signal
transduction due to the close relevance to chromatin remodeling, modulation
of IP3 levels, Ca2+ mobilization, and immune cell development. The in vivo
IP4 sensors in combination of other biosensors would realize simultaneous
monitoring of the bona fide behavior of multiple cellular second messengers
in the single-cell, which is currently underway in our laboratory. A rational
design of fluorescent biosensors for biologically relevant molecules in
the cell would accelerate the ubiquitous application of the real-time fluorescent
monitoring in the field of diagnosis and pharmacology. |
 |
| Structure-based design of a biosnsor for a cellular second messenger Ins(1,4,5)P4. |
 |
| The second messenger D-myo-inositol-1,4,5-trisphosphate (IP3) regulate intracellular Ca2+ concentration. Mapping real time intracellular IP3 concentration changes is an indispensable technique to elucidate the diversed cellular processes related to the IP3 production. We have developed a novel inositol 1,4,5-trisphosphate (IP3) sensor based on the pleckstrin homology (PH) domain from phospholipase C (PLC) . The environmentally sensitive fluorophore 6-bromoacetyl-2-dimethyl-aminonaphtalene was conjugated to the genetically introduced cysteine at the mouth of IP3-binding pocket, for enhanced IP3 selectivity and for rapid and direct visualization of intracellular IP3 > 0.5 M as fluorescence emission decrease. The probe, tagged with arginine-rich sequences for efficient translocation into various cell types, revealed a major contribution of Ca2+ influx to PLC-mediated IP3 production that boost Ca2+ release from endoplasmic reticulum. Thus, our IP3 probe was extremely effective to quantitatively assess real time physiological IP3 production via those pathways formed only in intact cellular configuration. |
 |
 |
 |
| An amino acid sequence for the PLC 1 PH domain (left) shows the positions
mutated to cysteine at 56R (blue), 58V (red) and 106N (yellow). Numbering
corrresponds to rat PLC 1 and constructs used here contain N-terminal Met.
A schematic illustration shows the structure of PLC 1 PH domain-IP3 complex
(right). Positions labeled by fluorophores at 56R, 58V and 106N are indicated
by CPK representation in blue, red and yellow, respectively, and IP3 is
shown by a wire-frame model. |
 |
| Novel in vivo Biosensors for IP4 Reveal Temporal IP4 Dynamics Inside Cells |
 |
| An optical sensor for IP4 was constructed by utilizing the PH domain of
GRP1 that possesses high affinity and selectivity to IP4. A unique cystein
residue, Cys15 or Cys82, was introduced to the GRP1 mutant followed by
labeling with the fluorophore to give 15F-IP4 and 82F-IP4, respectively. |
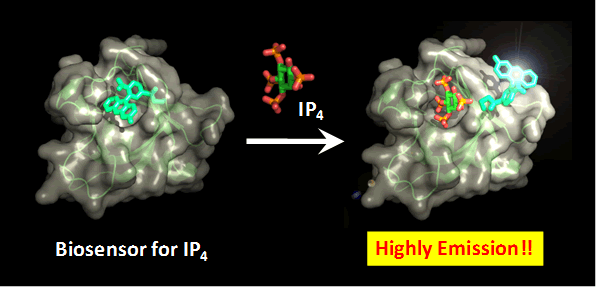 |
Figure. A schematic illustration shows the structure of biosensor for IP4
based on GRP1 PH domain and biosensor-IP4 complex. This biosensor exhibits
a highly fluorescent emission in response to IP4 binding.
|
 |
| The two IP4 sensors were next taken into HeLa cells by means of electroporation.
In HeLa cells, binding of histamine to H1 receptors activates PLC to produce
IP3, thus inducing Ca2+ release from the internal Ca2+ store. It is generally
accepted that inositol trisphosphate 3-kinase (IP3K) activated by Ca2+
ions phosphorylates IP3 to produce IP4. We monitored the time courses of
their fluorescence changes upon agonist stimulation of HeLa cells. Typical
traces observed for single-cell analysis under histamine stimulation (100
μM) by 15F-IP4 and 82F-IP4 successfully corresponded to the intracellular
IP4 concentration change (Figure 2B). Fluorescent sensors based on the
GRP1 PH domain exhibited appropriate affinity and specificity to IP4 and
distinct fluorescence responses upon target binding in single cells. These
IP4 sensors would serve as a tool to unveil a vital but yet unknown physiological
function of IP4. |
 |
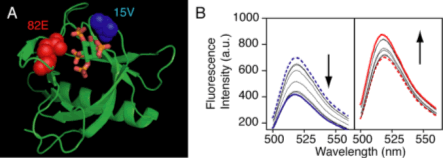 |
(A) A schematic illustration shows the structure of GRP1 PH domain-IP4 complex. Positions labeled by fluorescein at 15V and 82E are indicated by CPK representation in blue and red respectively, and IP4 is shown by a wire-frame model. (B) Emission spectra (initial: dashed, final: solid) show changes in intensity of the fluorophore-labeled PH domains 15F-IP4 (left) and 82F-IP4 (right) in the presence of increasing amount of IP4.
|
 |
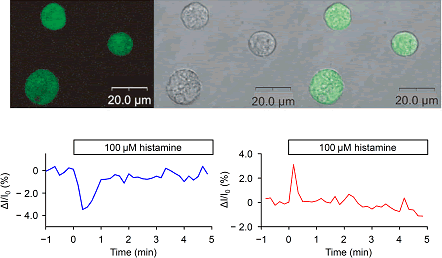 |
| (A) Confocal microscopic observation of 15F-IP4 in HeLa cells. Left, fluorescent
images. Middle, differential interference contrast (DIC) images of the
same cells. Right, merged images. (B) Time course of the production of
IP4 observed by temporal changes of 15F-IP4 (left) and 82F-IP4 (right)
fluorescence in a single cell under 100 μM histamine stimulation. |
 |
| These researches were supported by a Grant-in-aid for Scientific Research
from Ministry of Education, Science, Sports and Culture, Japan to T.M.
(No. 19021023, and No. 20241051). |
 |
| A Genetically Encoded Fluorescent Biosensor for IP4 Based on a Split PH
Domain |
 |
In order to visualize the behavior of secong messengers in a single live cell, the genetically encoded biosensors based on green fluorescent protein (GFP) represent the attractive and practical strategy.
We have demonstrated the functional reconstitution strategy based on a split pleckstrin homology (PH) domain by dissecting the PH domain and tethering a coiled coil module to each subunit. The reassembled split PH domain maintains binding selectively for IP3, similar to that of the native PLCδ1 PH domain. It is possible that the dissection strategy of the split PH domain would permit the target binding region of the PH domain to be contiguously fused to the essential residue for the fluorescence behavior of the cpGFP chromophore without sacrificing the intrinsic function of parent cpGFP and PH domain.
We designed and constructed a fluorescent sensor for an intracellular signal messenger IP4, from a split PH domain of Bruton’s tyrosine kinase (Btk) and a single cpGFP. The resulting split PH domain-cpGFP conjugate, Btk-cpGFP, exhibited bimodal absorption spectra corresponding to the protonated and deprotonated state of the chromophore in GFP. Addition of IP4 to Btk-cpGFP was accompanied by the decrease of 508 nm emission band by the excitation at 470 nm and the increase of the emission band by the excitation at 396 nm. As a result, the Btk-cpGFP realized the ratiometric fluorescence detection of IP4, and retained the ligand affinity and the selectivity of the original PH domain. |
 |
Schematic illustration shows a fluorescent biosensor for IP4 based on the
split Btk PH domain-cpGFP conjugate. The original N and C termini are linked
with a short peptide linker (orange), and the novel terminal of cpGFP (purple)
is fused to the split Btk PH domain (blue).
|
 |
1) T. Morii, K. Sugimoto, K. Makino, M. Otsuka, K. Imoto, Y. Mori, J. Am. Chem. Soc., 124, 1138 (2002)
2) K. Sugimoto, M. Nishida, M. Otsuka, K. Makino, K. Ohkubo, Y. Mori, T. Morii, Chem. Biol., 11, 475 (2004).
3) R. Sakaguchi, K. Tainaka, N. Shimada, S. Nakano, M. Inoue, S. Kiyonaka, Y. Mori, T. Morii, Angew. Chem., Int. Ed., 49, 2150 (2010).
4) R. Sakaguchi, T. Endoh, S. Yamamoto, K. Tainaka, K. Sugimoto, N. Fujieda, S. Kiyonaka, Y. Mori, T. Morii, Bioorg. Med. Chem., 17, 7381 (2009). |
 |
|
|