Integrated Research Center for Carbon Negative Science
Integrated Research Center for Carbon Negative Science
Professor : Toshiyuki NOHIRA
Associate Professor : Keiko KONDO
Junior Associate Professor : Rajendran ARIVAZHAGAN
Assistant Professor : Yutaro NORIKAWA
Assistant Professor : Peng LIN
Program-Specific Assistant Professor : Surachada CHUAYCHOB
To develop carbon-negative technologies, we are engaged in research to convert carbon dioxide into useful materials using renewable energy, biomass, etc.
https://icans.iae.kyoto-u.ac.jp/en/
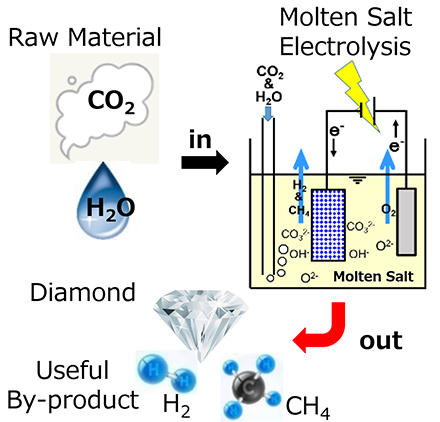
Production of Useful Substances from Carbon Dioxide Using Molten Salt Electrolysis
The conversion of carbon dioxide into useful substances is expected to contribute to the realization of a carbon-neutral society by 2050. If all the carbon dioxide generated from thermal power plants and steel-making plants is captured and converted into useful substances (Carbon Dioxide Capture and Utilization-CCU), it will greatly contribute to the carbon neutrality of our society. Furthermore, if carbon dioxide is captured from the atmosphere (Direct Air Capture-DAC) and converted into useful substances, it becomes carbon negative, which is even more significant. We have focused on molten salt electrolysis as a new method to convert carbon dioxide into useful substances. When carbon dioxide is injected into molten salt containing oxide ions (O2−), carbonate ions (O32−) are produced. When they are reduced at the cathode, various types of carbon are produced. Here, we are challenging to produce diamond, which is one of the most valuable allotropes of carbon. We are studying the optimum conditions for diamond formation by varying the temperature, composition of the molten salt, electrolytic potential, and other factors. So far, it has been found that diamond is formed by hydrogen generation from hydroxide ions (OH−) at the same time as carbon is deposited. Since OH− is produced by injecting water into the molten salt containing O2−, diamonds can be synthesized using only carbon dioxide and water as raw materials. The byproducts of this process are amorphous carbon, hydrogen gas, hydrocarbon gases (methane, etc.), and other useful substances, as well as non-toxic oxygen gas, making it a clean electrolysis method that does not emit hazardous substances.
Production of useful substances from carbon dioxide by molten salt electrolysis.
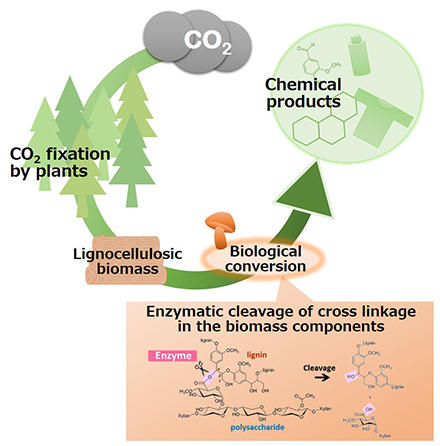
Development of Biological Conversion Processes of Plant Biomass
Carbon dioxide fixation proceeds in nature through photosynthesis by plants, where the carbon dioxide is converted to organic compounds and accumulated. Our society has been developed depending on various chemical products derived from fossil resources. Techniques to produce such chemical products from plant biomass enable long-term fixation of carbon, which accumulates in plants, as chemical products and leads to the realization of negative carbon emission. Lignocellulosic biomass such as wood is promising plant biomass that contains lignin, an aromatic resource alternative to fossil fuels. We are studying enzymes produced by wood-degrading microorganisms to reveal the mechanisms underlying the degradation of polysaccharides and lignin in the lignocellulosic biomass. On the basis of the findings, we are also working on the development of methodologies for separating, decomposing, and modifying each component of lignocellulosic biomass through biological processes using enzymes. Our research aims future development of a sustainable society that incorporates a material production system into the natural carbon cycle system.
Production of chemical products via biological processes.
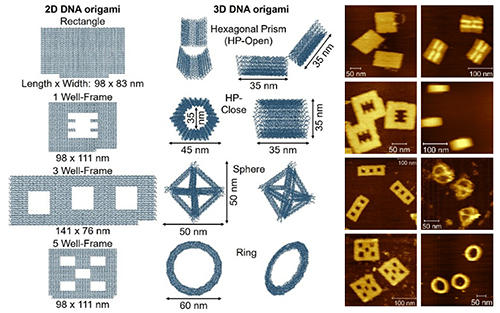
Development of stable DNA nanomaterials to perform cascaded reactions involving biomass-related enzymes
DNA origami is a technique for preparing nanomaterials by folding single-stranded DNA into predetermined shapes with sub-nanometer precision, enabling the creation of complex 2D and 3D structures. However, their widespread use is hindered by stability issues, particularly under thermal, mechanical, and biochemical stresses. These structures often melt at temperatures as low as 50-60°C and degrade under nuclease activity. To address these challenges, we developed two near-quantitative ligation methods: enzymatic ligation using dimethyl sulfoxide and enzyme-free chemical ligation with cyanogen bromide. These methods significantly improve structural stability, enhancing resistance to thermal treatment, electrophoresis, nuclease digestion, and cell lysates. We are also exploring stable DNA nanomaterials for handling biomass-related enzymes by arranging them with precision on these DNA nanomaterials in a distance-dependent manner. This approach aims to enhance enzymatic activity and enable cascaded enzymatic reactions for more efficient biomass conversion. These advancements improve DNA origami's stability and open new avenues for applications in enzyme catalysis, drug delivery, biomolecular sensing, and virus inhibition.
Schemes of the 2D and 3D DNA nanomaterials and their AFM images
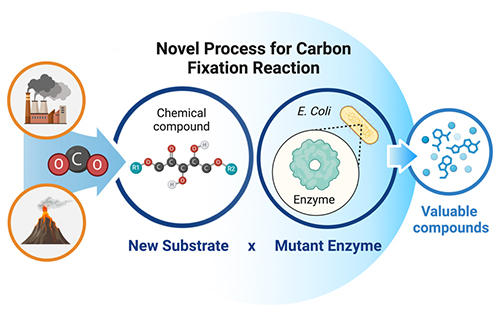
Enhanced Carbon Dioxide Utilization Through a Novel Process for Carbon Fixation Reaction
Carbon fixation, the process of converting inorganic carbon into organic compounds, plays a critical role in sustaining the biosphere. Advancing our ability to engineer carbon fixation reactions is essential for energy sustainability. By focusing on the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), the key enzyme in photosynthetic carbon fixation, our goal is to expand the substrate specificity of RuBisCO variants, enabling it to broaden its ability to convert carbon dioxide (CO₂) into valuable compounds. Our research focuses on Tk-RuBisCO, derived from Thermococcus kodakarensis KOD1, which exhibits remarkable thermostability, high carboxylase activity, and enhanced specificity at elevated temperatures. Its robust structural stability enables extensive mutagenesis, making it an excellent candidate for engineering novel CO₂ fixation pathways. To evaluate its potential for alternative CO₂ fixation reactions, we investigated its catalytic activity with both its natural substrate, ribulose-1,5-bisphosphate (RuBP), and other alternative substrates. Our findings highlight the feasibility of developing engineered RuBisCO variants for more efficient CO₂ conversion, paving the way for sustainable carbon utilization and industrial applications that support the transition to a carbon-negative future.
Enhanced Carbon Dioxide Utilization Through a Novel Process for Carbon Fixation Reaction.